Extracting limonene from oranges

Peel away the mysteries of this plant oil in a comprehensive class practical. Students can distil plant oils from oranges and discover the role of limonene in these fruits.
Introduction
This experiment demonstrates the extraction of plant oils. The peel of oranges is boiled in water and the oil produced (limonene) distilled in steam at a temperature just below 100°C, well below its normal boiling point.
The immiscible oil can then be separated. Direct extraction by heating would result in decomposition whereas steam distillation does not destroy the chemicals involved. This experiment can be conducted as a demonstration at secondary level as an introduction to some of the ideas about the extraction of plant oils.
This demonstration will take a full lesson of approximately 50 minutes, it can also be conducted as a class practical at key stages 4 and 5. Limonene is an unsaturated hydrocarbon which can be tested for using bromine water or potassium manganate (VII).
At a higher level, it is also a chiral compound and the experiment can lead to a discussion of optical enantiomers.
Equipment
Apparatus
- Eye protection
- Grater
- Bunsen burner
- Heat-resistant mat
- Tripod and gauze
- Orange x 2
- Thermometer up to 110°C
- Measuring cylinder, 100 cm 3
- Measuring cylinder, 50 cm 3
- Round bottomed flask, 250 cm 3
- Still head
- Thermometer pocket
- Condenser
- Receiver adapter
- Test tubes and bungs x 3
- Dropping pipette
- Anti-bumping granules
Chemicals
- Bromine water, no more than 0.2% v/v
- Potassium manganate(VII), 0.001 M
- Cyclohexene
- Distilled water, 100 cm 3
Health, safety and technical notes
- Read our standard health and safety guidance.
- Always wear eye protection.
- Always wear gloves when handling cyclohexene.
- Always use a gauze on the tripod or the orange will burn.
- Cyclohexene is Highly flammable, Harmful if swallowed and Toxic in contact with skin and cyclohexane is Highly flammable and a skin and respiratory irritant.(see CLEAPSS Hazcard HC045c).
- Bromine water is toxic and irritant. The concentration should not exceed 0.2% v/v (see CLEAPSS Hazcard HC015b).
- 0.001 M potassium manganate(VII) solution is of low hazard. This is a substitute for bromine water for student use (see CLEAPSS Hazcard HC081).
See below for the appropriate construction of a limonene distillation apparatus.
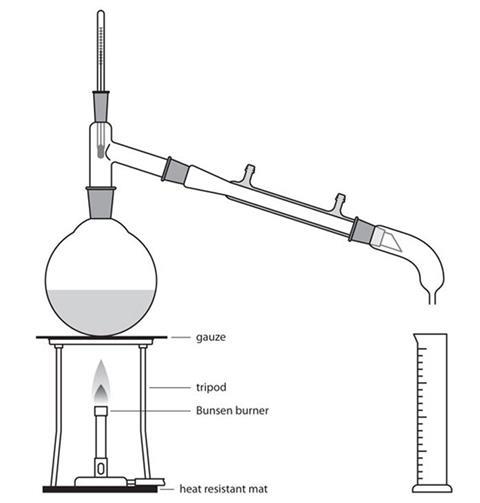
Source: Royal Society of Chemistry
Diagram of limonene distillation
Procedure
Stage 1
- Grate the outer orange coloured rind of two oranges and add to 100 cm 3 of distilled water in the 250 cm 3 round bottomed flask. Add anti-bumping granules to the round bottomed flask.
- Set up the distillation apparatus as shown above.
- Heat the flask so that distillation proceeds at a steady rate, approximately one drop per second of distillate. (Note: take care not to let the liquid in the round bottomed flask boil too strongly).
- Collect approximately 50 cm 3 of distillate in the measuring cylinder. The oil layer will be on the surface.
- Using a dropping pipette, carefully remove the oil layer into a test tube for the next stage.
Stage 2
Odour
- Cautiously smell the extracted oil by wafting the fumes towards the nose. Do not breathe in directly from the test tube.
Action of bromine water
- Measure out approximately 1 cm 3 of bromine water into each of three test tubes.
- Add a few drops of the limonene oil to one test tube, a few drops of cyclohexane to another, and a few drops of cyclohexene to the third. Place in the bungs and agitate. If the bromine water is decolourised the molecule contains double bonds.
- 0.001 M potassium manganate(VII) can be substituted for the bromine water for class use. However, students need to know the action of bromine water.
Notes
- Limonene (1-methyl-4-prop-1-en-2-yl-cyclohexene) is an unsaturated hydrocarbon, classed as a terpene.
- At room temperature, it is a colourless oily liquid with the smell of oranges. Its molecular formula is C10H16 and its boiling point is 176°C.
- Limonene is a chiral molecule with two optical isomers (enantiomers).
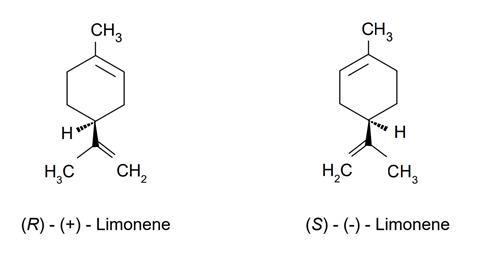
The chemical structure of two different forms of limonene
- The major biological form dlimonene, the (R)-enantiomer, is used in food manufacture and medicines.
- It is also used as a fragrance in cleaning products, a botanical insecticide, and due to its flammability, a potential biofuel.
- The (S)-enantiomer, l-limonene, is also used as a fragrance but has a piney, turpentine odour.
- It is possible to allow students to observe the optical activity of chiral molecules by comparing saturated glucose solution with distilled water in a polarimeter.
- The amount of oil extracted varies considerably with the variety, season and storage of the oranges, however, it is always possible to extract sufficient oil.
- Do not distil more than 50% of the initial volume of water, or solid “jam” will form in the flask which is difficult to remove.
- Limonene (1-methyl-4-prop-1-en-2-yl-cyclohexene) is an unsaturated hydrocarbon, classed as a terpene.
- At room temperature, it is a colourless oily liquid with the smell of oranges.
- Its molecular formula is C10H16 and its boiling point is 176°C.
Downloads
Extracting limonene from oranges - teacher notes
Additional information
This practical is part of our Chemistry for non-specialists collection. This experiment was written by Andrew Thompson on behalf of the RSC.




